3 Lab 3—Mineral identification
The geological definition of a mineral is, “a naturally occurring crystalline solid substance, generally inorganic, with a specific chemical composition.” (Press and Siever, 2004)
PHYSICAL PROPERTIES OF MINERALS
The most commonly used physical properties used to identify minerals are 1. color, 2. luster, 3. hardness, 4. streak, 5. cleavage or fracture, and 6. crystal habit. In this lab, you will describe and classify mineral samples using each of these properties.
Color is often the first property used when initially trying to identify a mineral. However, color can be misleading when determining a specific mineral and it should not be relied upon too heavily. Also, remember to note the clarity of the color in the mineral. Descriptive terms for clarity include transparent, translucent, and opaque.
Luster describes the way light interacts with the surface of a mineral or rock. Due to light reflecting off a mineral, it can appear metallic or non-metallic. Metallic minerals are completely opaque—you cannot see through them—and have the luster of polished metal. Non-metallic lusters are further subdivided into several categories as listed below.
Metallic
Non-metallic
Vitreous (glassy)—often transparent or translucent, e.g., quartz or calcite
Dull (earthy)—not at all shiny, e.g., kaolinite
Resinous—appearing like resin, chewing gum, or smooth plastic, e.g., amber
Waxy—looks like wax, e.g., jade or cryptocrystalline quartz
Greasy—having an appearance like fat or grease, may also feel greasy to the touch, e.g., opal
Pearly—reflectionlikepearlsresultsfromperfectcleavage,e.g., muscovite
Silky—made up of thin crystals that look like silk fibers, when coarser in appearance this can be a fibrous luster, e.g., ulexite
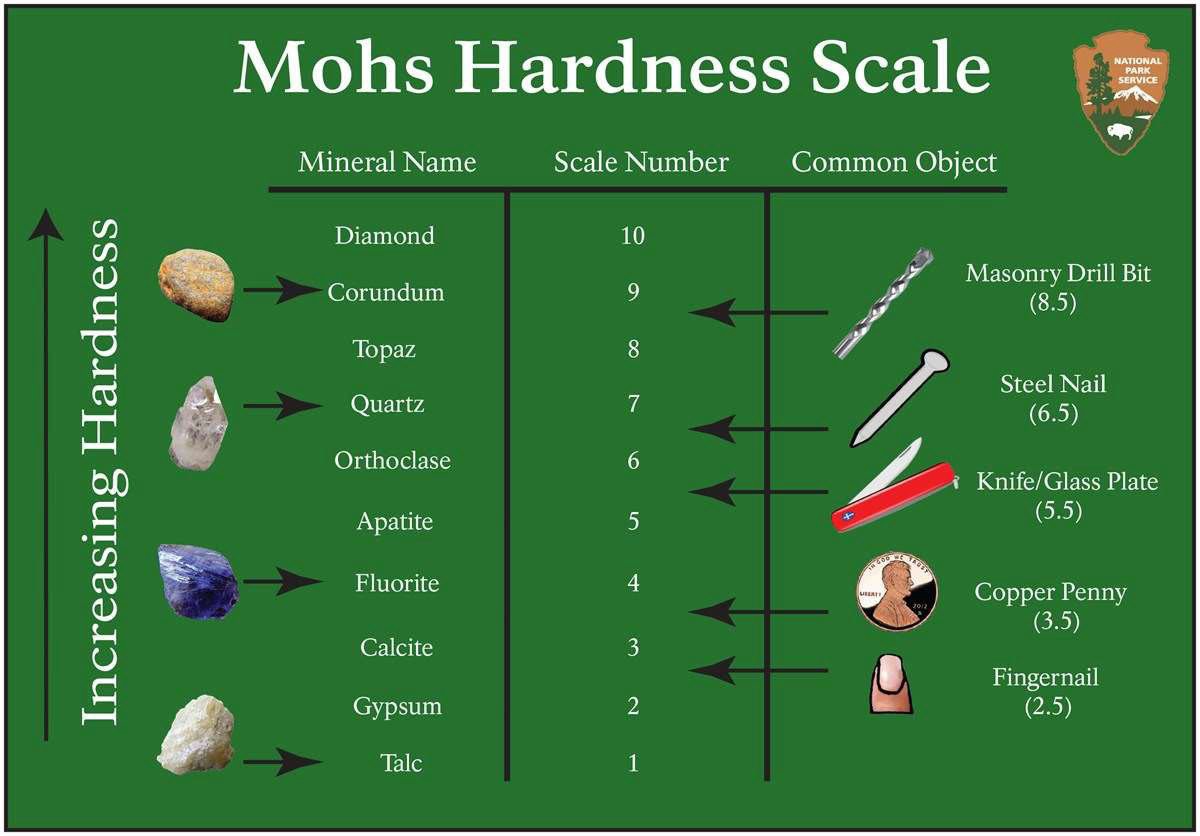
Mohs Hardness (H) is a measure of the resistance of a mineral to scratching (not breaking, as in along cleavage planes), or the resistance a smooth surface offers to abrasion.
Table 2.1 Mohs Scale of Hardness (NPS, 2019)
Refers to the color of a powdered mineral. This is more consistent than the color of a whole crystal and therefore provides a more reliable feature for mineral identification. You can obtain this by scratching the mineral on an unpolished piece of white porcelain called a streak plate.
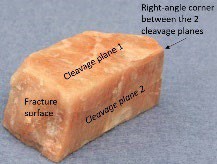
Cleavage is the tendency of a mineral to break along smooth parallel flat surfaces, revealing the underlying atomic structure of the mineral. See Figure 2.1 for illustration of cleavage. Remember to include these aspects of the mineral’s cleavage:
How many cleavage planes are apparent?
How strong/apparent is cleavage? (excellent, good, poor, no cleavage)
At what angle do cleavage plains meet? (90° or 60° & 120° are most common, other angles are possible)
Fracture is the result of a mineral having no cleavage planes, and represents a mineral’s tendency to display random, irregular breakage, or breakage along non- parallel planes. Commonly observed fracture patterns include conchoidal and splintery fracture. Conchoidal minerals break along surfaces that then display concentric circle patterns, while rocks with splintery fracture break along multiple surface with the resulting pattern resembling splintered wood.
|
Number of Cleavages and their directions |
Name and description of how the mineral breaks |
Shape of broken pieces with example mineral |
Illustration of cleavage directions |
|
No cleavage (fractures only) |
No parallel broken surfaces; may have conchoidal fracture (like glass) |
quartz |
None (no cleavage) |
|
1 cleavage plane |
Basal (book) cleavage “Books” that split along flat sheets |
muscovite, biotite |
|
|
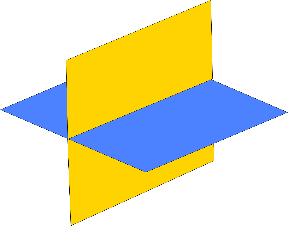
2 cleavage planes Intersect at or near 90° |
Prismatic cleavage Elongated forms that break along short rectangular sections |
orthoclase feldspar, plagioclase feldspar, pyroxene |
|
|
2 cleavage planes Do not intersect at 90° |
Prismatic cleavage Elongated forms that break along short parallelogram cross sections |
amphibole (hornblende) 56° and 124° |
|
|
3 cleavage planes Intersect at 90° |
Cubic cleavage Shapes made of cubs and parts of cubes |
halite, galena |
|
|
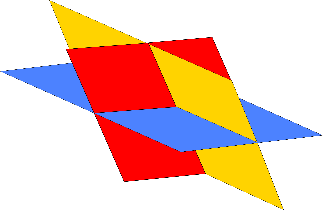
3 cleavage planes Do not intersect at 90° |
Rhombohedral cleavage Shapes made of rhombohedrons and parts of rhombohedrons |
calcite and dolomite 75° and 105° |
|
|
4 cleavage planes Intersect at 71° and 109° (may have secondary cleavage at 60° and 120°) |
Octahedral cleavage Shapes made of octahedrons and parts of octahedrons |
fluorite |
|
|
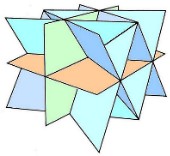
6 cleavage planes Intersect at 60° and 120° |
Dodecahedral cleavage Shapes made of dodecahedrons and parts of dodecahedrons |
sphalerite |
|
Figure 2.1 Cleavage in Minerals
Crystal habits are the distinctive form or shape that a mineral may take in different geologic settings. Habits describe the ideal growth form of the mineral (with well- formed crystal faces), provided the mineral has the free space to complete unhindered growth. Here are some examples of crystal habit: cubic, octahedral, tabular (rectangular), acicular (long, slender, needle- like), fibrous, dendritic (branching like a tree), or botryoidal (smooth, bulbous). For a longer list with illustrations, check out https://en.wikipedia.org/wiki/Crystal_habit.
Magnetism
Taste / Odor / Feel
Tenacity—how a mineral resists breakage. Is the mineral Elastic? Flexible? Brittle? Malleable?
Reaction to Acid—Carbonate minerals, such as calcite, tend to react with hydrochloric acid (HCl). This reaction is referred to as effervescence. It is important to note whether the mineral effervesces strongly with acid or weakly.
Specific Gravity—How heavy does the mineral feel? Is it particularly heavy or dense? Is it surprisingly light?
Striations—fine, straight “scratches” visible on the surface of specific minerals, such as plagioclase feldspar
MINERAL IDENTIFICATION FLOW CHART
To identify each mineral sample, start by observing the mineral’s luster: metallic, non-metallic with a lighter color, or non-metallic with a darker color. In the mineral descriptions, listed within parentheses are the rock types in which that mineral is commonly found: I = igneous, M = metamorphic, S = sedimentary.
Is the mineral’s luster metallic?
If NO, go to 2.
If YES, continue with Metallic Luster Chart:
|
Streak Color |
Mineral Color |
Other properties |
Mineral Name |
Chemical Formula |
|
black, gray or greenish black |
bright metallic to sub- metallic lead- gray |
3 cleavage directions, perfect at 90°, very heavy (ρ=7.6), cubic crystal habit, H=2.5 (M) |
Galena |
PbS |
|
black to dark gray |
strongly magnetic, conchoidal or irregular fracture, ρ=5.2, H=5.5–6.5 (I, S) |
Magnetite |
Fe3O4 |
|
|
steel gray |
smudges fingers, shiny, slippery, ρ=2, H=1–2, luster may be dull (I, M) |
Graphite |
C |
|
|
brass yellow |
cubic crystal habit, striations common, common in granular aggregates, uneven fracture, ρ=4.8–5, H=6–6.5 |
Pyrite |
FeS2 |
|
|
greenish black |
golden yellow |
may tarnish iridescent purple, may be weakly magnetic, ρ=3.5–4, H=4.3 |
Chalcopyrite |
CuFeS2 |
|
brown to reddish brown |
red-brown, steel gray, or black |
granular, fibrous, or micaceous, brittle, uneven fracture ρ=5.26, H=5–6 (S) |
Hematite |
Fe2O3 |
|
yellow or brown |
yellow, brown, or black |
hard, structureless, or radial fibrous masses; can be cubic as pseudomorph after pyrite, ρ=2.7–4.3, H=4–5.5 (S) |
Limonite |
FeO(OH)· nH2O |
|
metallic copper red |
copper red, tarnishes to dull brown, black, or green |
often found as distorted masses or extremely distorted crystals, ρ=8.94–8.95, H=2.5–3 (I, M, S) |
Native Copper |
Cu |
Non-metallic luster: Is the mineral’s color lighter or darker?
If non-metallic luster with lighter color, go to 3.
If non-metallic luster with darker color, go to 4.
Non-metallic luster with lighter color: Does the mineral scratch glass?
If YES, use this chart:
Non-metallic luster, lighter color, harder than glass:
|
Demonstrates cleavage? |
Other properties |
Mineral Name |
Chemical Formula |
|
cleavage prominent |
2 cleavage planes at almost 90°, light to dark pink, blocky, luster = pearly, vitreous, or resinous, ρ=2.55–2.63, H=6 (I, M, S) |
Orthoclase feldspar |
KAlSi3O8 |
|
2 cleavage planes at almost 90°, white to gray to bluish gray, blocky, striations on some cleavage planes, ρ=2.6–2.8, H=6–6.5 (I, M, S) |
Plagioclase feldspar |
NaAlSi3O8 to CaAl2Si2O8 |
|
|
cleavage absent |
conchoidal fracture, glassy, transparent to translucent, hexagonal crystal habit, well-formed crystals common, varieties named by color: milky, smoky, rose, and amethyst, vitreous luster, ρ=2.65, H=7 (I, M, S) |
Quartz |
SiO2 |
|
conchoidal fracture, translucent to opaque, white, yellow, gray, black, or brown, sometimes banded, luster waxy or dull, ρ=2.6, H=6.5–7 (I, M, S) |
Cryptocrystalline quartz |
SiO2 |
|
If NO, use this chart:
Non-metallic luster, lighter color, softer than glass:
|
Demonstrates cleavage? |
Other properties |
Mineral Name |
Chemical Formula |
|
cleavage prominent |
perfect cubic cleavage, salty taste, colorless, white or pale orange, forms cubes, soluble in water, ρ=2.168, H=2.5 (S) |
Halite |
NaCl |
|
perfect cleavage in 1 direction, poor in 2 others, white, transparent, nonelastic, wide variety of habits (needles, small crystals, curling “flowers”), luster = vitreous, silky, pearly, or dull, ρ=2.3, H=2 (S) |
Gypsum |
CaSO4•2H2O |
|
|
perfect rhombohedral cleavage, at about 75° (squashed cube), effervesces in HCl, white or colorless, pale yellow, rarely gray or blue, transparent to opaque, luster = vitreous, resinous, pearly, or waxy, ρ=2.7, H=3 (S, M) |
Calcite |
CaCO3 |
|
|
4 good cleavage directions—octahedral; colorless, green, purple, yellow or brown, glassy; transparent to translucent; cubic crystal habit, luster vitreous or dull, ρ=3.2–3.6, H=4 (I, M, S) |
Fluorite |
CaF2 |
|
|
one perfect cleavage plane, thin flexible sheets, colorless to light yellow, transparent, luster = vitreous, silky, or pearly, ρ=2.8–2.9, H=2.5 (M, S) |
Muscovite |
KAl3AlSi3O10(OH) |
|
|
gray, green, pink or white, greasy or soapy feel, luster = resinous, waxy, greasy, or pearly, one direction of cleavage forms thin scales, foliated or compact masses, ρ=2.58– 2.83, H=1 (M) |
Talc |
Mg3Si4O10(OH)2 |
|
|
cleavage absent |
crystals so small no cleavage is visible, white to red, earthy masses, soft, becomes plastic when moistened, earthy odor, ρ=2.6, H=2–2.5 (I, M, S) |
Kaolinite |
Al2Si2O5(OH)4 |
|
crystal habit: well-shaped hexagonal crystals, which may be prismatic, dipyramidal, and stubby; colorless, white, yellow, brown, gray, red, pink, purple, blue, green; transparent to translucent, vitreous, ρ=3.16–3.22, H=5 (I, M) |
Apatite |
Ca5(PO4)3(Cl/F/OH) |
|
|
bright yellow to yellow-brown, greasy feel, exhibits a strong “rotten-egg” odor, soluble in warm water, ρ=2.07, H=1.5–2.5 (I, S) |
Sulfur |
S |
|
Non-metallic luster with darker color: Does the mineral scratch glass?
If YES, use this chart:
Non-metallic luster, darker color, harder than glass:
|
Demonstrates cleavage? |
Other properties |
Mineral Name |
Chemical Formula |
|
cleavage prominent |
2 cleavage planes at almost 90°, black to dark green, short, prismatic, 8-sided crystals, ρ=3.2–3.6, H=5.5–6 (I) |
Augite |
(Ca,Na) (Mg,Fe,Al,Ti) (Si,Al)2O6 |
|
2 cleavage planes ~60° and 120°, dark green to black or brown, long prismatic 6-sided crystals, ρ=3–3.4, H=6 (I, M) |
Hornblende |
(Ca,Na)2–3 (Mg,Fe,Al)5 (Al,Si)8O22 (OH,F)2 |
|
|
2 cleavage planes at almost 90°, gray to dark blue- gray, blocky, striations on some cleavage planes, ρ=2.6–2.8, H=6–6.5 (I, M, S) |
Plagioclase feldspar |
NaAlSi3O8 to CaAl2Si2O8 |
|
|
cleavage absent |
bright to dark green, glassy luster, usually in granular masses, brittle, conchoidal fracture, transparent to translucent, ρ=3.2–4.5, H=6.5–7 (I) |
Olivine |
(Mg,Fe)2SiO4 |
|
red color most common, green, yellow, brown possible, glassy to resinous luster, conchoidal to uneven fracture, commonly in 12-sided dodecahedral crystals, ρ=3.1–4.3, H= 6.5–7.5 (M) |
Garnet |
(Ca,Mg,Fe,Mn)3 (Al,Fe,Cr)2 (SiO4)3 |
|
|
conchoidal fracture, gray to gray-black, vitreous luster, transparent to translucent, hexagonal crystal habit, well-formed crystals common, ρ=2.65, H=7 (I, M, S) |
Smoky quartz |
SiO2 |
|
If NO, use this chart:
Non-metallic luster, darker color, softer than glass:
|
Demonstrates cleavage? |
Other properties |
Mineral Name |
Chemical Formula |
|
cleavage prominent |
one perfect cleavage plane, flexible and elastic when in thin sheets, brown to black, ρ=2.7–3.3, H=2.5–3 (M, S) |
Biotite |
K(Mg,Fe)3 AlSi3O10(F,OH)2 |
|
green to very dark green, 1 cleavage direction, foliated or scaly masses, ρ=2.6–3.3, H=2–2.5 (M) |
Chlorite |
(Mg,Fe)3(Si,Al)4O10 (OH)2•(Mg,Fe)3(OH)6 |
|
|
yellowish brown, resinous luster, cleavage in 6 directions, yellowish brown or nearly white streak, ρ=3.9–4.1, H=3.5–4 (I, M, S) |
Sphalerite |
ZnS |
|
|
4 good cleavage directions; colorless, green, purple, yellow or brown, glassy; transparent to translucent; cubic crystal habit, ρ=3, H=4 (I, M, S) |
Fluorite |
CaF2 |
|
|
cleavage absent |
red to red-brown streak, dull to earthy luster, ρ=5.26, H=5–6 (S) |
Hematite |
Fe2O3 |
|
Yellowish-brown streak, yellowish brown to dark brown; hard, structureless, or radial fibrous masses; can be cubic as pseudomorph after pyrite, ρ=2.7–4.3, H=4–5.5 (S) |
Limonite |
FeO(OH)·nH2O |
|
Mineral Identification Table for Lab 2
|
Sample Number |
Luster |
Hardness (H) |
Cleavage or Fracture |
Color |
Streak |
Crystal Habit and/or other properties |
Mineral Name |
|
1 |
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
9 |
|
|
|
|
|
|
|
|
Sample Number |
Luster |
Hardness (H) |
Cleavage or Fracture |
Color |
Streak |
Crystal Habit and/or other properties |
Mineral Name |
|
10 |
|
|
|
|
|
|
|
|
11 |
|
|
|
|
|
|
|
|
12 |
|
|
|
|
|
|
|
|
13 |
|
|
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
|
15 |
|
|
|
|
|
|
|
|
16 |
|
|
|
|
|
|
|
|
17 |
|
|
|
|
|
|
|
|
Sample Number |
Luster |
Hardness (H) |
Cleavage or Fracture |
Color |
Streak |
Crystal Habit and/or other properties |
Mineral Name |
|
18 |
|
|
|
|
|
|
|
|
19 |
|
|
|
|
|
|
|
|
20 |
|
|
|
|
|
|
|
|
21 |
|
|
|
|
|
|
|
|
22 |
|
|
|
|
|
|
|
|
23 |
|
|
|
|
|
|
|
|
24 |
|
|
|
|
|
|
|
|
25 |
|
|
|
|
|
|
|
|
26 |
|
|
|
|
|
|
|
REFERENCES
Chinellato, Matteo, “Sphalerite, Lengenbach Quarry, Fäld, Binn, Goms, Valais, Switzerland,” https://www.mindat.org/photo-312483.html; last access: 2022-06-29.
Cristofono, Peter, 2006, “Muscovite, Palermo No. 1 Mine, Groton, Grafton County New Hampshire, USA,” https://www.mindat.org/photo-73662.html; last access: 2022-06-29.
Cronin, Vince, 2002, “Amphibole Data: Specimen 2,” https://croninprojects.org/Vince/PhysGeoLab/amphibole2.jpg; last access: 2022-06-29.
Earle, Steven, 2019, “Figure 2.6.5 Cleavage and fracture in potassium feldspar,” Physical Geology – 2nd Edition. Victoria, B.C.: BCcampus, https://opentextbc.ca/physicalgeology2ed/chapter/2-6-mineral- properties/; last access: 2022-06-29.
Earle, Steven. (2019), “Figure 2.6.6 . . . cleavage planes in the mineral fluorite. . .,” Physical Geology – 2nd Edition. Victoria, B.C.: BCcampus, https://opentextbc.ca/physicalgeology2ed/chapter/2-6- mineral-properties/; last access: 2022-06-29.
Gillman, Joe, 2016, “Galena: Missouri’s Official State Mineral,” https://dnr.mo.gov/document- search/galena-missouris-official-state-mineral-pub0658/pub0658; last access: 2022-06-29.
Minot, Henry, “Calcite (Var: Iceland Spar), Rockland, Knox County, Maine, USA,” https://www.mindat.org/photo-533325.html; last access: 2022-06-29.
NPS, (National Park Service). “Mohs Harness Scale.” Accessed July 18, 2019. https://www.nps.gov/articles/mohs-hardness-scale.htm.
Press, Frank, Raymond Siever, John Grotzinger, and Thomas H Jordan. Understanding Earth. Macmillan, 2004.
Rizzo, Russel G. and Cal Neva Mineral Company, “Quartz (Var: Amethyst), Nangarhar, Afghanistan,” https://www.mindat.org/photo-112839.html; last access: 2022-06-29.
Rygel, M.C., CC BY-SA 3.0, “6 directions of cleavage,” https://commons.wikimedia.org/w/index.php?curid=10127224; last access: 2022-06-29.