11 The Endocrine Response to Exercise
Learning Objectives
-
Identify the three classes of hormones based on their chemical makeup.
-
Discuss how the chemical composition of hormones affects their transport in the blood and interaction with tissues.
-
Describe how hormones like insulin facilitate glucose uptake in cells.
-
Explain the G protein-coupled receptor mechanism and its significance in cellular responses.
-
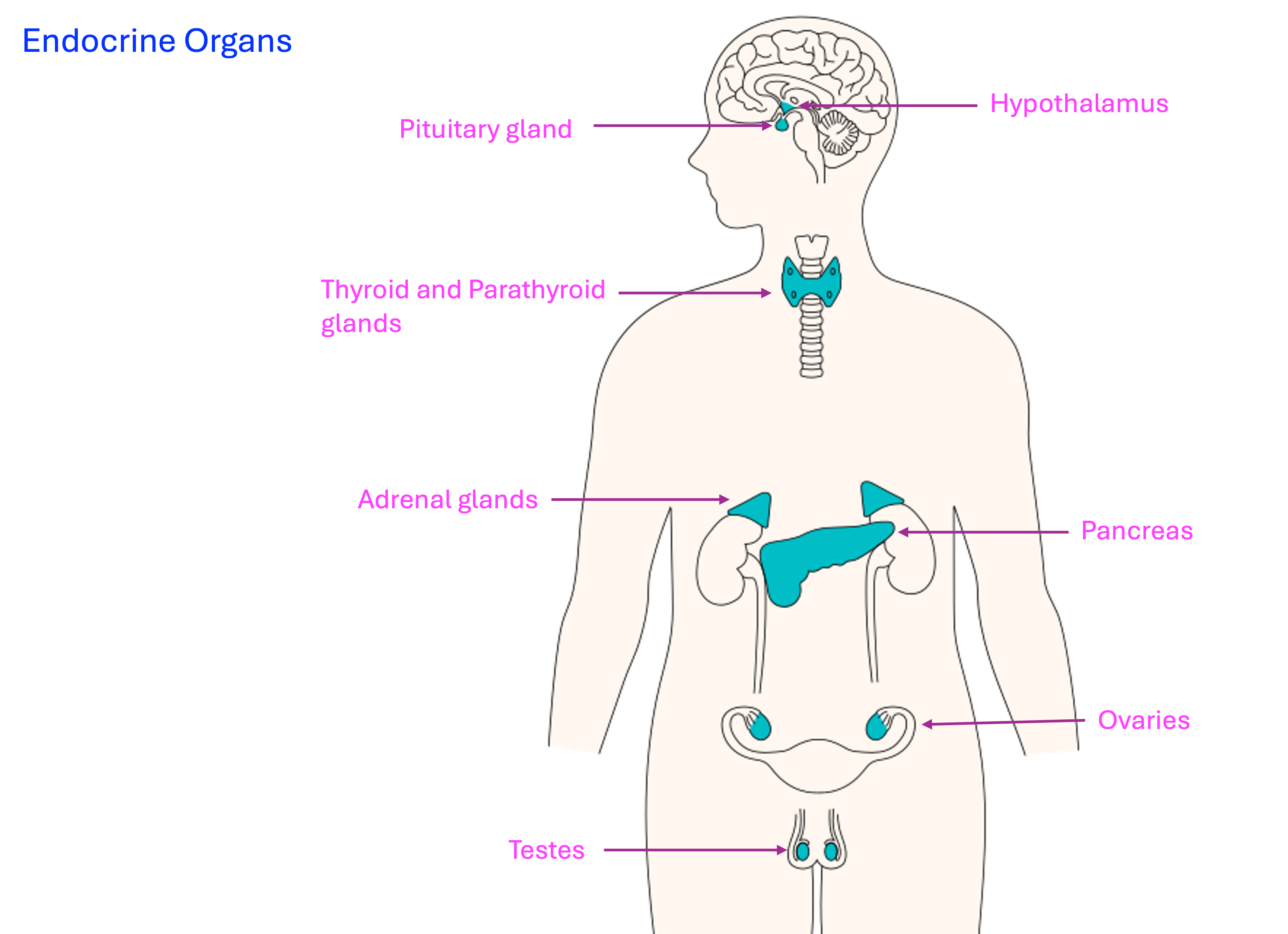
Identify the endocrine glands most affected by exercise and the hormones they secrete.
-
Explain how changes in plasma volume and osmolality during exercise influence hormone secretion.
-
Describe the primary hormones secreted by the adrenal medulla and cortex and their roles during exercise.
-
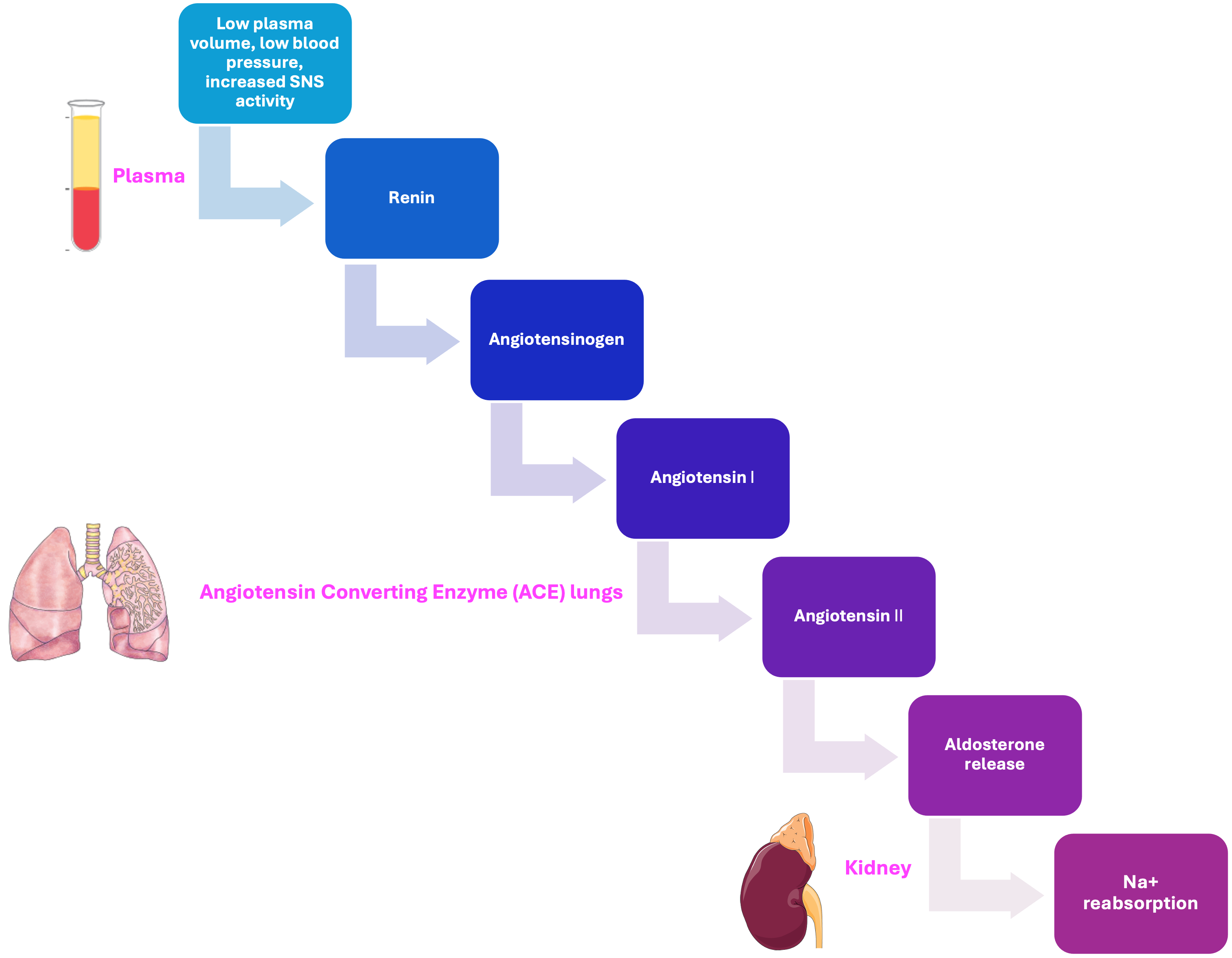
Explain the renin-angiotensin-aldosterone system and its importance in maintaining plasma volume during exercise.
-
Explain the functions of testosterone and estrogens in the body and their influence on exercise performance.
-
Describe how anabolic hormones like testosterone, GH, insulin, and IGF-1 contribute to muscle hypertrophy and remodeling during resistance training.
-
Compare the acute and chronic hormonal responses to resistance training and their significance.
Introduction
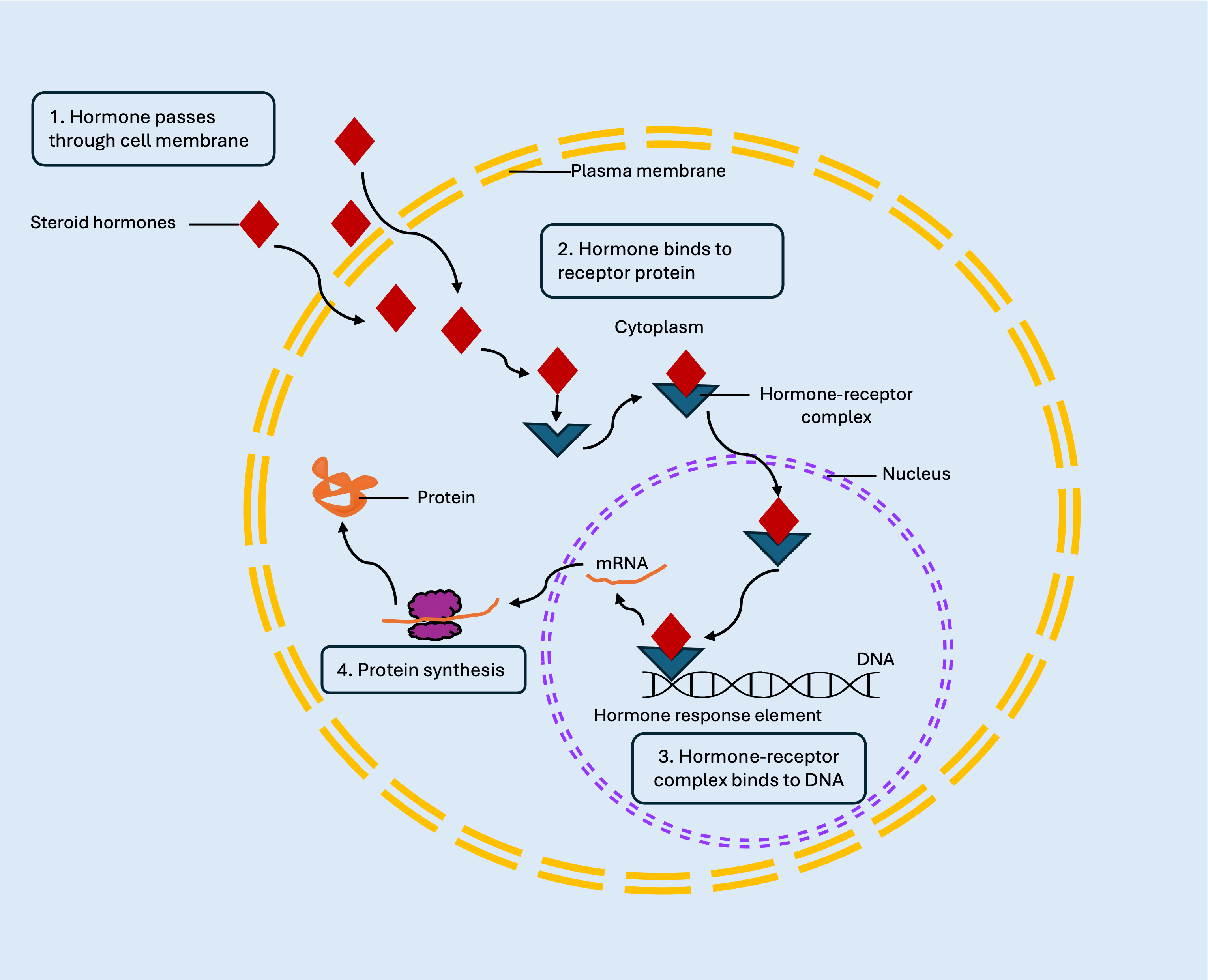
Categories of Hormones
Altering DNA activity
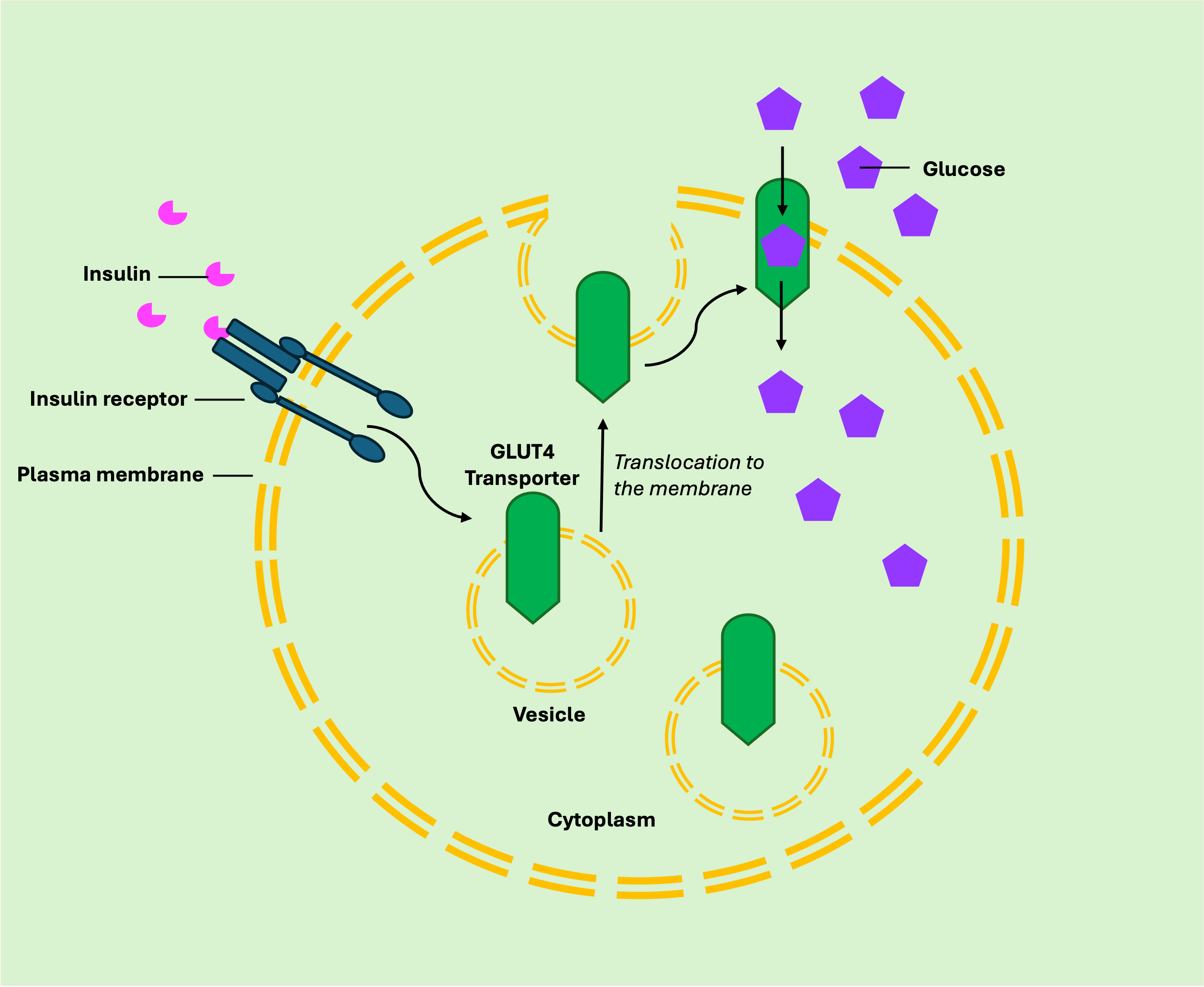
Altering membrane transport activity
-
Insulin binds to the alpha subunit of a tyrosine kinase receptor located outside the cell.
-
Binding causes the beta subunits inside the cell to phosphorylate themselves.
-
The activation of these subunits leads to the movement of glucose transporters, known as GLUT4 transporters, to the cell membrane.
-
GLUT4 transporters then facilitate the entry of glucose into the cell.
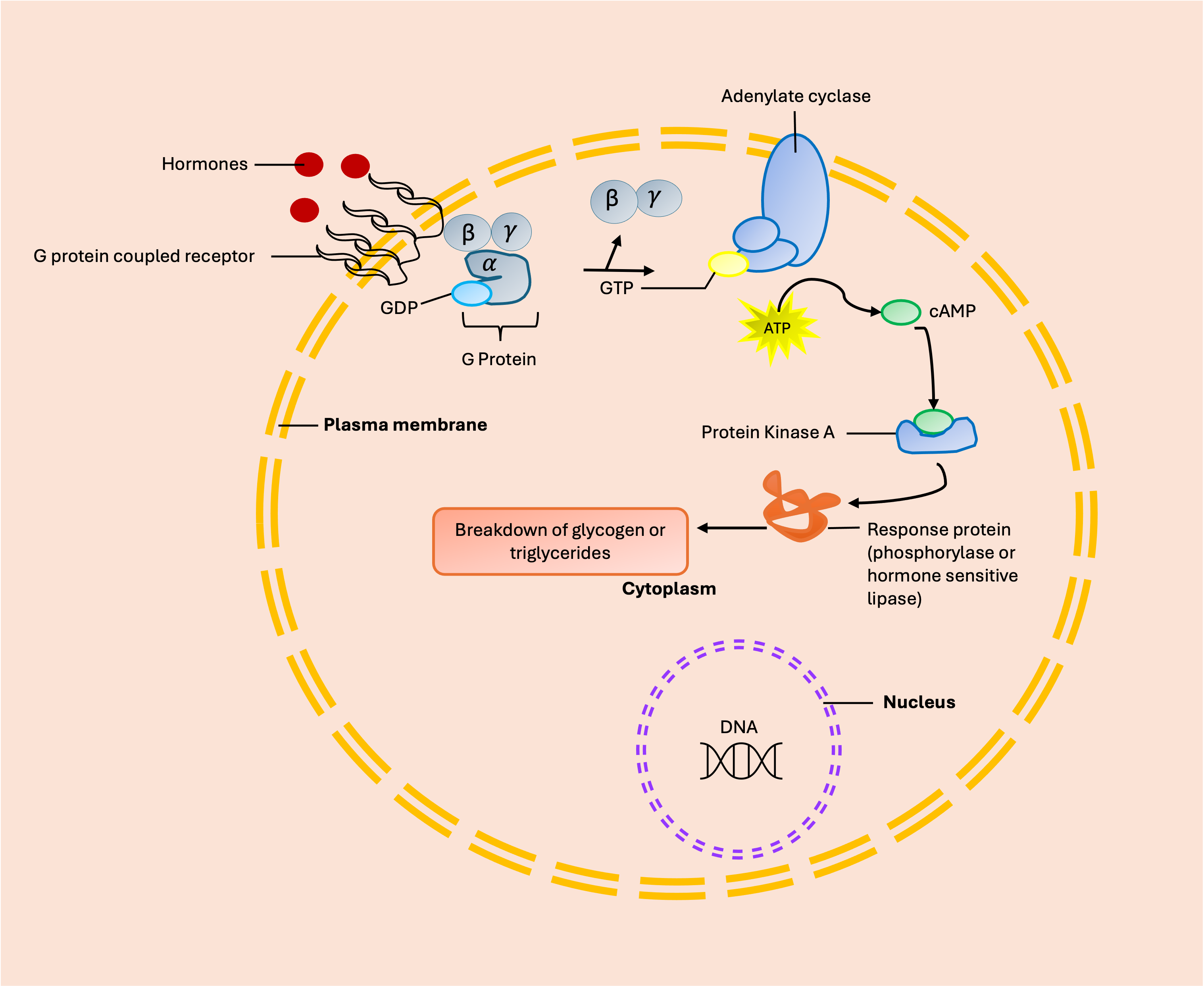
Second Messenger Receptor Mechanism
Secretion of Hormones and the General Exercise Response
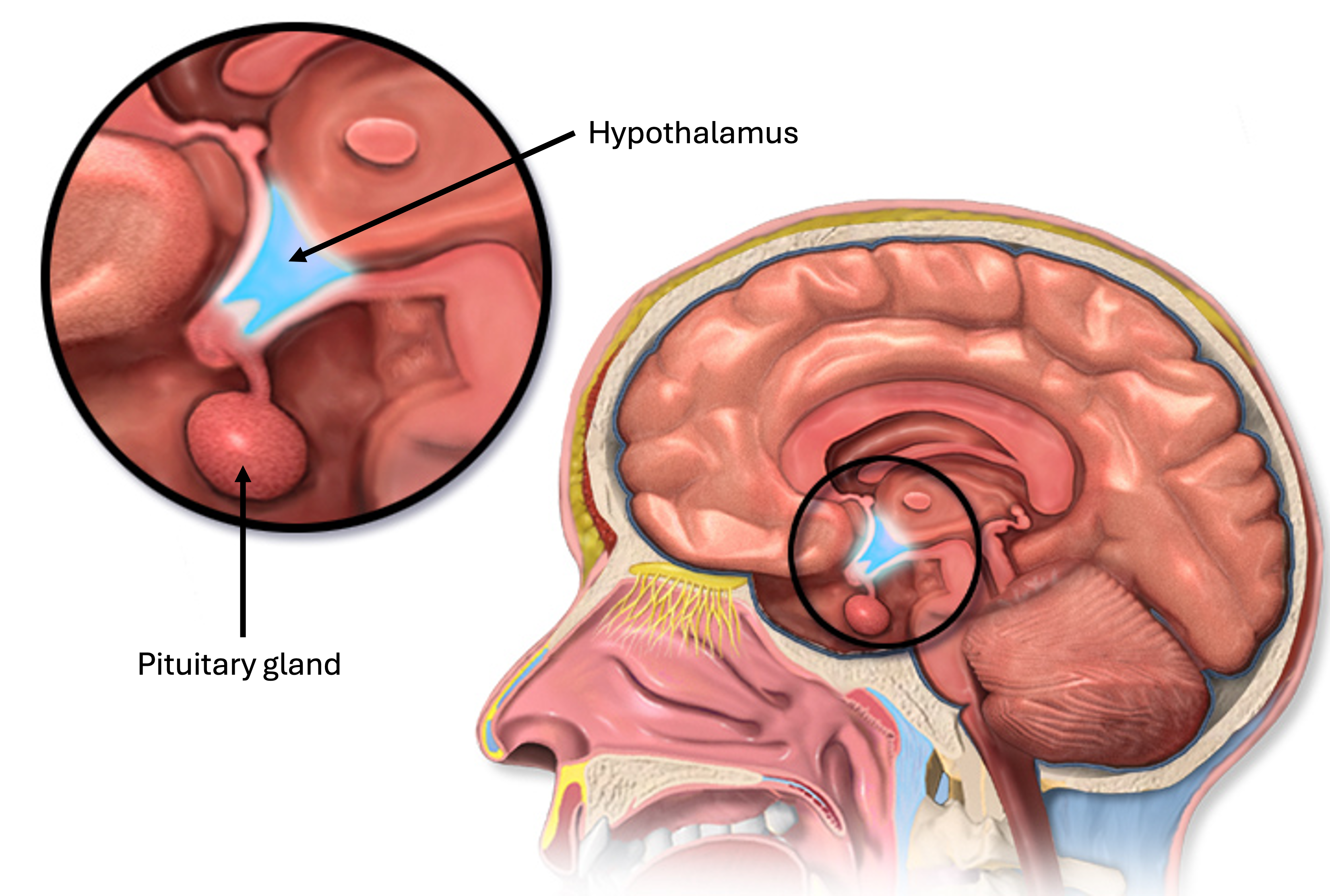
Hypothalamus and the Pituitary Gland
Anterior pituitary and its hormones
-
Adrenocorticotrophic Hormone (ACTH): Stimulates the production and secretion of cortisol in the adrenal cortex in response to stress.
-
Luteinizing Hormone (LH): Stimulates the production of testosterone and estrogen.
-
Thyroid-Stimulating Hormone (TSH): Regulates the rate and secretion of thyroid hormones.
-
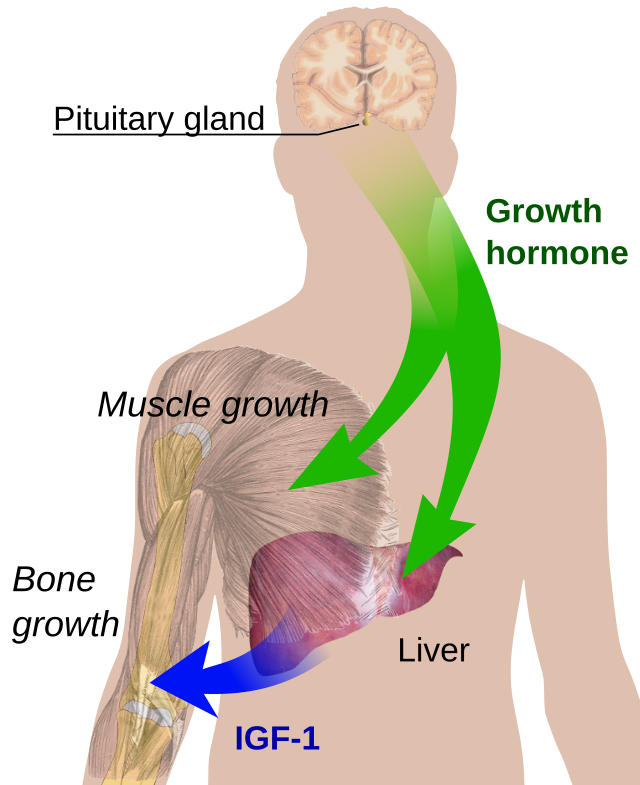
Growth hormone (GH) stimulates the release of insulin-like growth factors (IGFs) from the liver and other tissues, promoting protein synthesis and tissue growth.
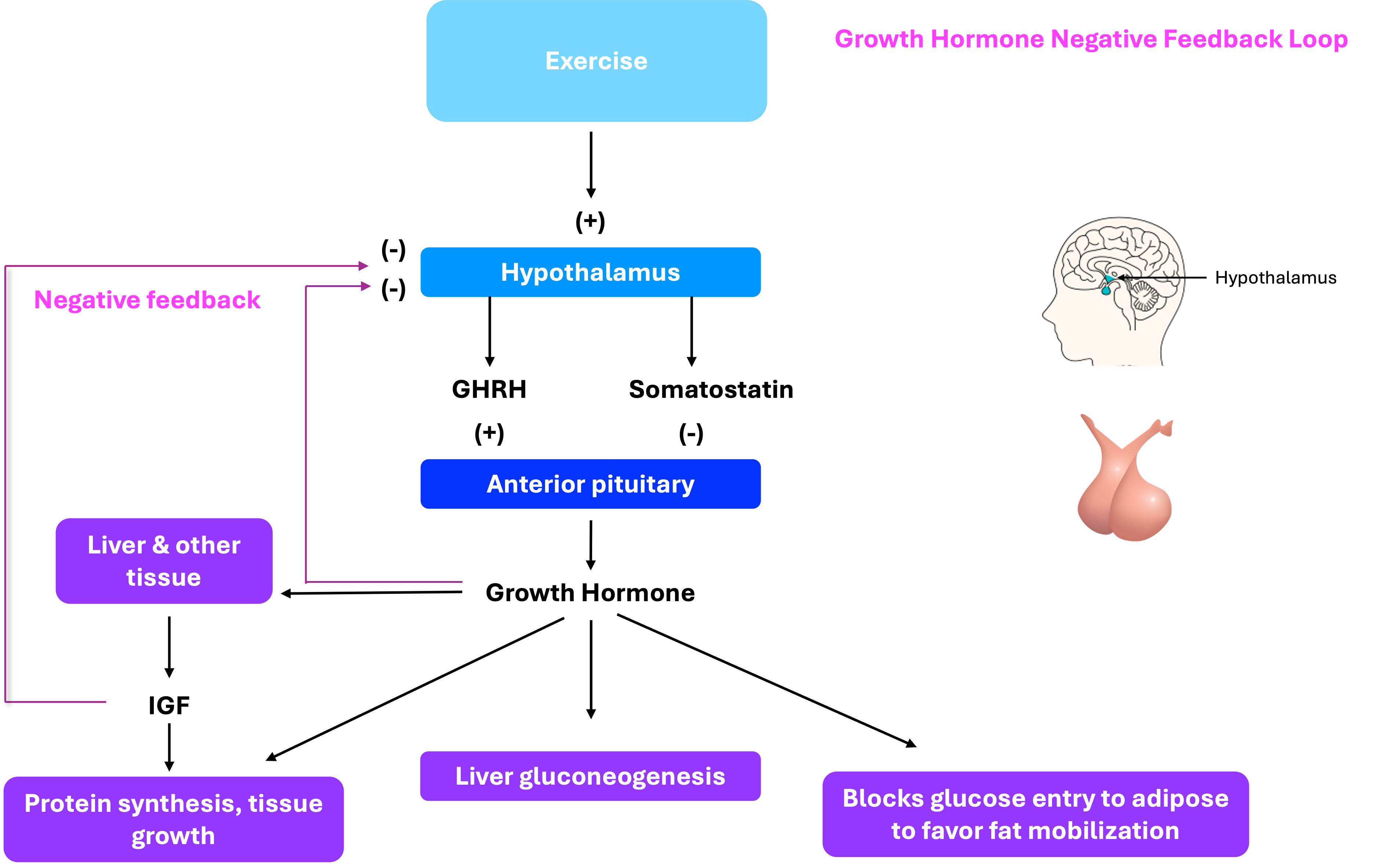
Growth Hormone (GH) and its regulation
Posterior pituitary gland
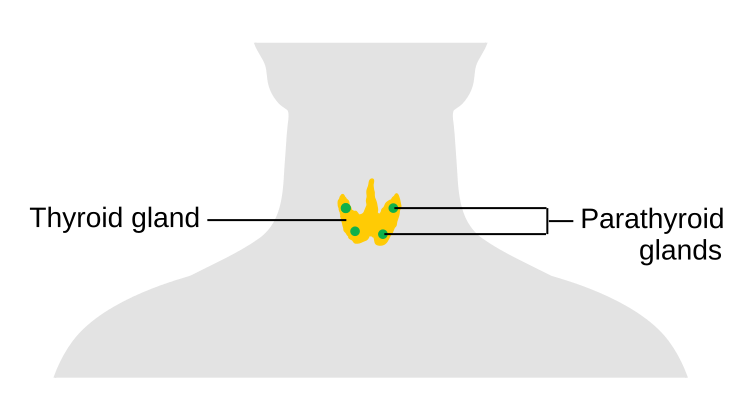
Thyroid and Parathyroid Glands
Calcium regulation and parathyroid hormone
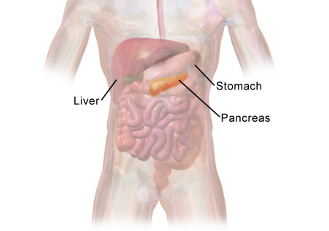
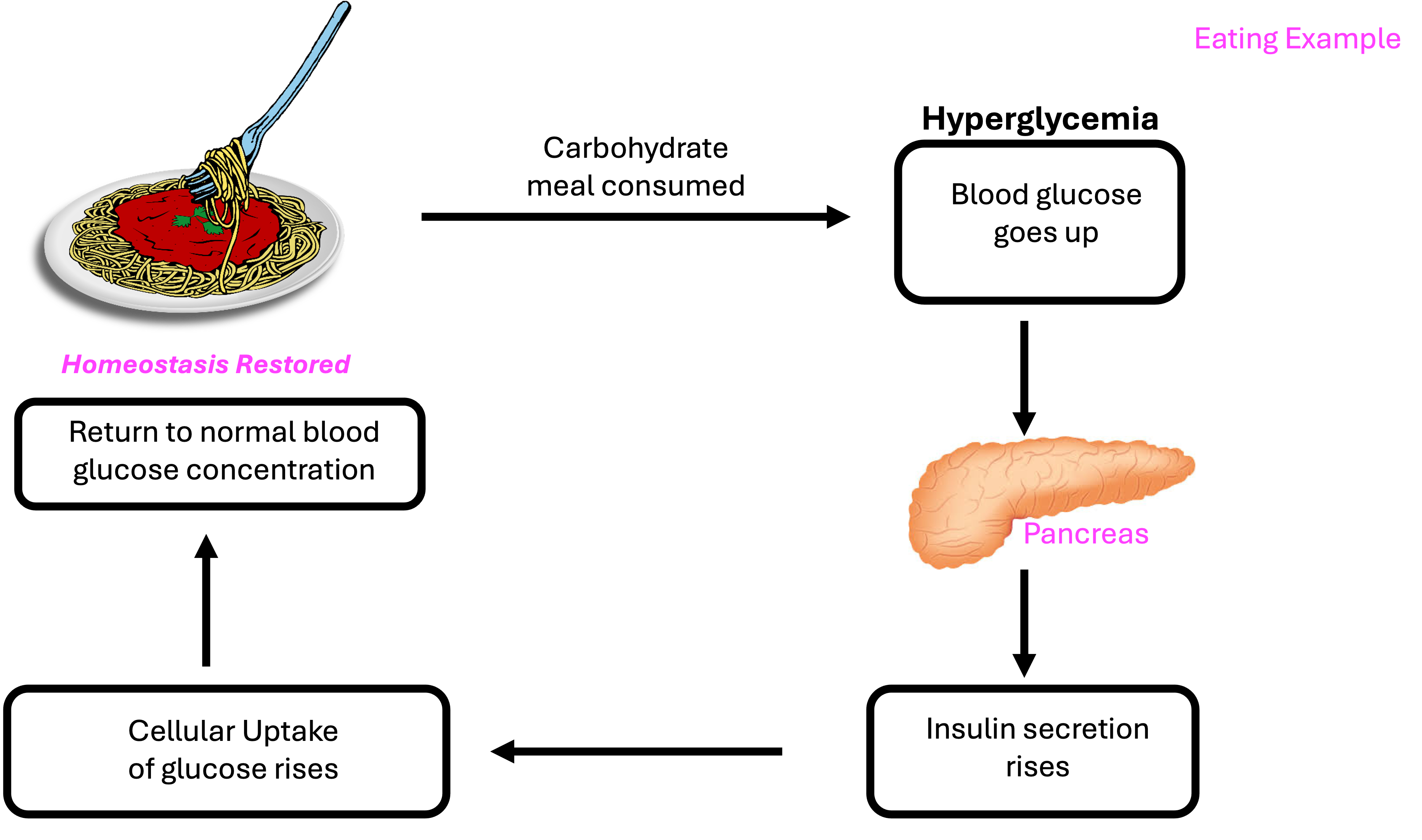
Pancreas
Insulin and its role in glucose regulation
Insulin and glucagon responses during exercise
Adrenal Glands
Adrenal medulla and its hormones
Adrenal cortex and its hormones
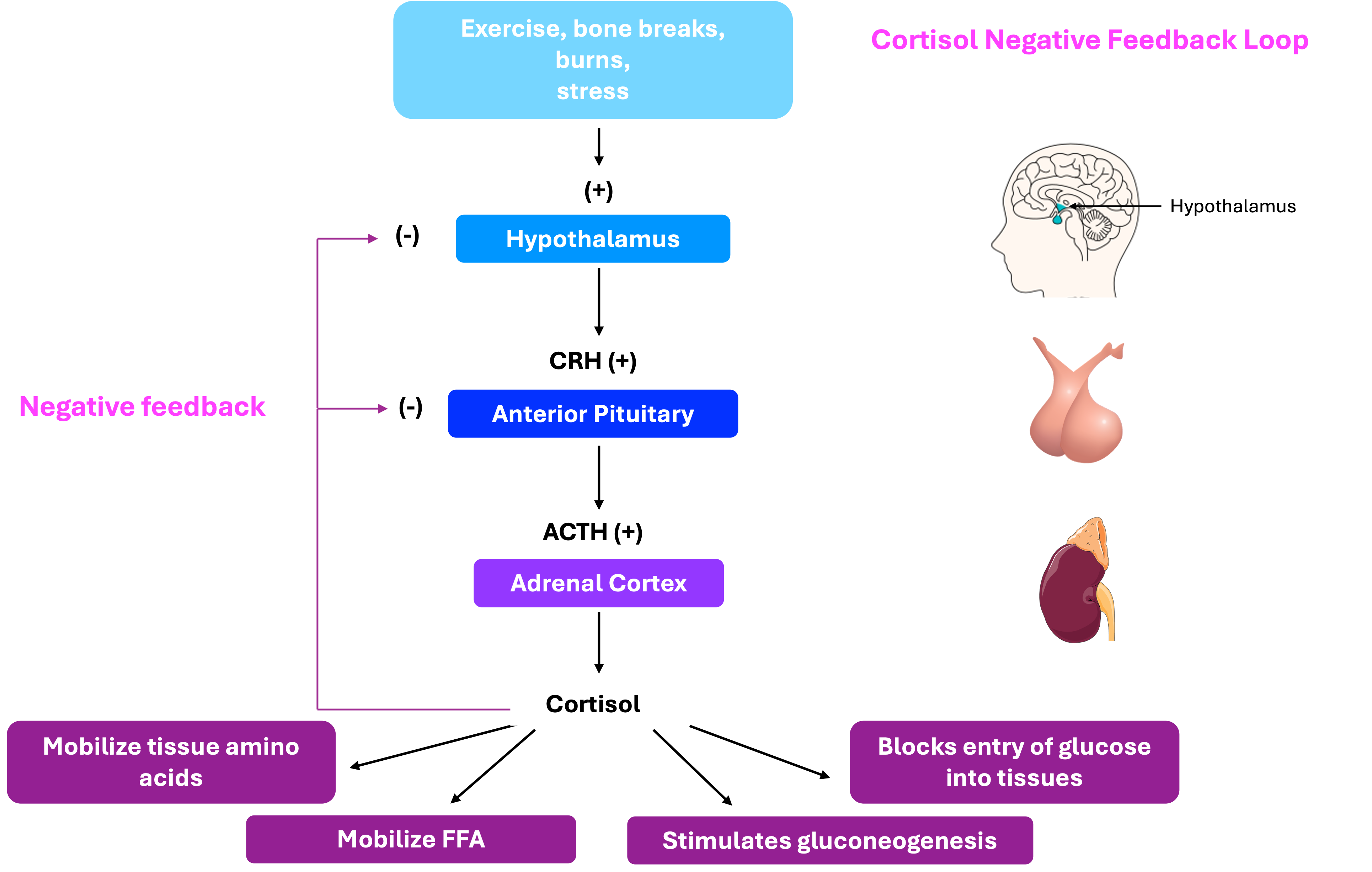
Cortisol and its role in metabolism
Testes and Ovaries
|
Gland
|
Hormone
|
Action
|
Control Factors
|
Stimuli
|
Acute Exercise Effect
|
Chronic Exercise Effect
|
|---|---|---|---|---|---|---|
|
Anterior pituitary
|
Growth Hormone (GH)
|
Growth, FFA mobilization, gluconeogenesis; decreases glucose uptake
|
GH-releasing hormone; somatostatin
|
Exercise; stress; low blood glucose
|
Increase
|
Attenuated response at same rate of work
|
|
Thyroid-stimulating hormone (TSH)
|
Increases T3 and T4 production and secretion
|
TSH-releasing hormone
|
Low plasma, T3 and T4
|
Increase
|
No known effect
|
|
|
Adrenocorticotrophic hormone (ACTH)
|
Increases cortisol synthesis and secretion
|
ACTH-releasing hormone
|
Stress; bone breaks; heavy exercise; burns
|
Increase
|
Attenuated response
|
|
|
Follicle-stimulating hormone (FSH); luteinizing hormone (LH)
|
Female: Estrogen and progesterone production and ovum development
Male: testosterone production and sperm development
|
Hypothalamic gonadotrophic-releasing hormone
Females: plasma estrogen and progesterone
Males: plasma testosterone
|
Firing of neurons in the hypothalamus
|
Small or no change
|
No known effect
|
|
|
|
Endorphins
|
Block pain in opiate receptors in the brain
|
ACTH-releasing hormone
|
Stress
|
Increases in exercise > or = 70% VO2max
|
Unknown
|
|
Posterior pituitary
|
Antidiuretic hormone (ADH) (vasopressin)
|
Decrease water loss at kidney; increases peripheral resistance
|
Hypothalamic neurons
|
Plasma volume; plasma osmolality
|
Increase
|
Attenuated response
|
|
Thyroid
|
Triiodothyronine (T3); thyroxine (T4)
|
Increase metabolic rate, mobilization of fuels, growth
|
TSH; plasma T3 and T4
|
Low T3 and T4
|
Increase in “free” T3 and T4
|
Increase turnover of T3 and T4 at same work rate
|
|
Calcitonin
|
Decreases plasma calcium
|
Plasma calcium
|
Elevated plasma calcium
|
Unknown
|
Unknown
|
|
|
Parathyroid hormone
|
Increase plasma calcium
|
Plasma calcium
|
Low plasma calcium
|
Increase
|
Unknown
|
|
|
Adrenal cortex
|
Cortisol
|
Increases gluconeogenesis, FFA mobilization, heart rate, stroke volume, and peripheral resistance
|
ACTH
|
Stress; bone breaks; heavy exercise; burns
|
Increases in heavy exercise; decreases in light exercise
|
Slight increase
|
|
|
Aldosterone
|
Increases potassium secretion and sodium reabsorption at kidney
|
Plasma potassium concentration and renin-angiotensin system
|
Low blood pressure, low plasma volume, elevated K+
|
Increase
|
Unchanged
|
|
Adrenal medulla
|
Epinephrine (E) (80%); norepinephrine (NE) (20%)
|
Increases glycogenolysis, FFA mobilization, heart rate, stroke volume, and peripheral resistance
|
Output of baroreceptors; glucose receptor in hypothalamus; brain and spinal centers
|
Low blood pressure and blood pressure; stress and emotion
|
Increase
|
Attenuated response
|
|
Pancreas
|
Insulin
|
Increases glucose amino acid, and FFA uptake into tissues
|
Plasma glucose and amino acid concentration; autonomic nervous system
|
Elevated plasma glucose and amino acid concentration; decreased E and NE
|
Decrease
|
Attenuated response
|
|
Testes
|
Testosterone
|
Protein synthesis; secondary sex characteristics
|
FSH and LH (ICSH)
|
Increased FSH and LH
|
Small increase
|
Resting levels decreased
|
|
Ovaries
|
Estrogens and progesterone
|
Fat deposition; secondary sex characteristics; ovum development
|
FSH and LH
|
Increased FSH and LH
|
Small increase
|
Resting levels may be decreased in trained women
|
Endocrine Responses to Resistance Exercise
Acute Hormonal Response to Resistance Training
Insulin and Insulin-Like Growth Factor-1 (IGF-1)
IGFs are small polypeptide hormones secreted by the liver in response to GH-stimulated DNA synthesis. Their main role is to increase protein synthesis following resistance training, resulting in muscle hypertrophy. Recent evidence suggests that IGF-1 increases gene and protein expression due to the stretch and tension associated with resistance training. However, the response is delayed until GH-stimulated synthesis and secretion from the liver occur, with peak values not reached until 16-28 hours post-GH release [15]. Thus, the short-term responses of IGF-1 remain unclear. Chronically, training volume and intensity are important for chronic resting IGF-1 adaptations.
Catecholamines
Glucocorticoids
|
Hormone
|
Acute response
|
Chronic response
|
|---|---|---|
|
Testosterone
|
Increase in men, no change or elevation in women
|
No change or inconsistent results
|
|
Growth Hormone
|
Increases in both sexes
|
No change
|
|
Cortisol
|
Increases in both sexes
|
No change or inconsistent results
|
|
IGF-1
|
No change
|
Increases with high volumes and intensities
|
|
Insulin
|
Decreases without supplementation
|
No change
|
|
Catecholamines (Epinephrine, Norepinephrine, Dopamine)
|
Increase
|
Unclear
|
Chapter Summary
-
Introduction to Exercise and Homeostasis: Exercise disrupts homeostasis, prompting acute and chronic changes regulated by the nervous and endocrine systems. Hormones play a vital role in mobilizing fuel, stimulating protein synthesis, and initiating muscle hypertrophy.
-
Categories of Hormones: Hormones are classified into amino acid derivatives, peptides/proteins, and steroid hormones. Their chemical makeup influences their transport in the blood and interaction with tissues.
-
Mechanisms of Hormone Action: Hormones modify cellular activity through altering DNA activity, membrane transport, and activating second messenger proteins. Examples include insulin’s role in glucose uptake and the G protein-coupled receptor mechanism.
-
Secretion of Hormones During Exercise: The chapter highlighted the endocrine glands most affected by exercise, including the hypothalamus, pituitary gland, thyroid, parathyroid glands, and pancreas. Hormonal regulation during exercise involves complex feedback mechanisms to maintain plasma glucose and calcium levels.
-
Adrenal Glands: The adrenal medulla and cortex secrete hormones like epinephrine, norepinephrine, cortisol, and aldosterone, which are crucial for stress response, metabolism, and maintaining plasma volume during exercise.
-
Testes and Ovaries: Testosterone and estrogens are essential for reproductive function and influence exercise performance. The chapter discussed their roles in muscle hypertrophy, secondary sex characteristics, and the impact of menstrual cycle irregularities on bone health.
-
Endocrine Responses to Resistance Exercise: Resistance training induces significant hormonal changes, particularly in anabolic hormones like testosterone, GH, insulin, and IGF-1, which are critical for muscle growth and remodeling. The chapter also covered the role of catecholamines and glucocorticoids in exercise. Resistance training elicits hormonal changes essential for muscle hypertrophy, force production, and energy liberation. Short-term effects depend on training intensity, volume, muscle mass targeted, recovery, and frequency, while long-term adaptations are minimal but related to training volume and intensity.
Scholarly Questions
-
How does exercise act as a stressor on the body, and what are the acute and chronic changes it induces?
-
What roles do the nervous and endocrine systems play in maintaining homeostasis during exercise?
-
What are the three classes of hormones based on their chemical makeup, and how does this affect their transport and interaction with tissues?
-
How do steroid hormones differ from peptide/protein hormones in terms of their mechanism of action?
-
Describe the process by which insulin facilitates glucose uptake in cells.
-
Explain the G protein-coupled receptor mechanism and its significance in hormone action.
-
Which endocrine glands are most affected by exercise, and what hormones do they secrete?
-
How do changes in plasma volume and osmolality during exercise influence hormone secretion?
-
What are the primary hormones secreted by the adrenal medulla and cortex, and what are their roles during exercise?
-
How does the renin-angiotensin-aldosterone system help maintain plasma volume during exercise?
-
What are the functions of testosterone and estrogens in the body, and how do they influence exercise performance?
-
Discuss the impact of menstrual cycle irregularities on bone health in female athletes.
-
How do anabolic hormones like testosterone, GH, insulin, and IGF-1 contribute to muscle hypertrophy and remodeling during resistance training?
-
What are the acute and chronic hormonal responses to resistance training, and how do they differ?
-
Why is the acute hormonal response to resistance training more critical than chronic changes in resting hormone concentrations?
-
How do catecholamines and glucocorticoids influence exercise performance and muscle adaptation?
- Convertino VA, Keil LC, and Greenleaf JE. Plasma volume, renin, and vasopressin responses to graded exercise after training. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 54: 508-514. ↵
- Bouassida A, Latiri I, Bouassida S, Zalleg D, Zaouali M, Feki Y, et al. Parathyroid hormone and physical exercise: a brief review. J Sport Science and Medicine. 5: 367-374, 2006. ↵
- Terjung R. Endocrine Response to Exercise. New York, NY: Macmillan, 1979. ↵
- Jensen J, Oftebro H, Breigan B, Johnsson A, Ohlin K Meen HD, et al. Comparison of changes in testosterone concentrations after strength and endurance exercise in well trained men. European Journal of Applied Physiology and Occupational Physiology. 63:467-471, 1991. ↵
- Vogel RB, Books CA, Ketchum C, Zauner CW, and Murray FT. Increase of free and total testosterone during submaximal exercise in normal males. Medicine and Science in Sports and Exercise. 17: 119-123, 1985. ↵
- Schoenfeld BJ. Postexercise hypertrophic adaptations: a reexamination of the hormone hypothesis and its applicability to resistance training program design. Journal of Strength and Conditioning Research. 27: 1720-1730, 2013b. ↵
- Oosthusysee T, and Bosch AN. The effect of the menstrual cycle on exercise metabolism. Sports Medicine. 40: 207-227, 2010. ↵
- Kraemer RR, Francois M, Webb ND, Worley JR, Rogers SN, Norman RL, et al. No effect of menstrual cycle phase on glucose and glucoregulatory endocrine responses to prolonged exercise. European Journal of Applied Physiology. 113: 2401-2408, 2013. ↵
- Xanne A, and Janse de Jonge X. Effects of the menstrual cycle on exercise performance. Sports Medicine. 33: 833-851, 2003. ↵
- Redman LM, and Louchs AB. Menstrual disorders in athletes. Sports Medicine. 35: 747-755, 2005. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
- Kraemer WJ, Ratamess NA, Hormonal Responses and Adaptations to Resistance Exercise and Training. Review Article. Sports Medicine, 2005. 35(4): p. 339-361. ↵
Chemical messengers produced by endocrine glands and released into the bloodstream to regulate various physiological processes
A class of hormones derived from cholesterol that are lipid-soluble and capable of passing through cell membranes to bind with intracellular receptors. They regulate a wide range of physiological processes including metabolism, immune response, salt and water balance, and reproductive functions.
Also called "peptide hormones", polypeptide hormones are composed of short chains of amino acids that are water-soluble and typically bind to receptors on the surface of target cells, triggering intracellular signaling cascades rather than directly altering gene expression. Peptide hormones regulate a wide range of physiological functions including growth, metabolism, and reproduction.
A peptide hormone produced by the beta cells of the pancreatic islets (Islets of Langerhans). It plays a central role in regulating blood glucose levels by promoting the uptake of glucose into cells, especially in muscle and adipose tissue, and by stimulating glycogen synthesis in the liver.
Guanine nucleotide-binding proteins, are molecular switches that play a critical role in transmitting signals from cell surface receptors to intracellular effectors. They are activated when a signaling molecule (e.g., a hormone or neurotransmitter) binds to a G protein-coupled receptor (GPCR), causing the G protein to exchange GDP for GTP. This activation triggers downstream signaling pathways that regulate various cellular responses.
A small but crucial region of the brain located below the thalamus and above the pituitary gland. It serves as a major control center for the autonomic nervous system and the endocrine system, maintaining homeostasis by regulating body temperature, hunger, thirst, sleep, and emotional activity.
Often called the “master gland,” is a small, pea-sized endocrine gland located at the base of the brain, beneath the hypothalamus. It regulates many vital body functions by secreting hormones that control other endocrine glands.
A portion of the pituitary gland that produces hormones such as growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin.
A portion of the pituitary gland that stores and releases oxytocin and antidiuretic hormone (ADH), which are produced by the hypothalamus.
A peptide hormone produced and secreted by the anterior pituitary gland in response to corticotropin-releasing hormone (CRH) from the hypothalamus. ACTH stimulates the adrenal cortex, particularly the zona fasciculata, to produce and release glucocorticoids, primarily cortisol.
A glycoprotein hormone secreted by the anterior pituitary gland that plays a key role in regulating reproductive function.
A glycoprotein hormone produced by the anterior pituitary gland that stimulates the thyroid gland to produce and release thyroxine (T₄) and triiodothyronine (T₃). These thyroid hormones regulate metabolism, growth, and development.
Also known as somatotropin, is a peptide hormone secreted by the anterior pituitary gland. It plays a vital role in stimulating growth, cell reproduction, and regeneration. GH promotes the growth of bones and muscles, increases protein synthesis, and mobilizes fat stores for energy. It also influences metabolism by increasing blood glucose levels and stimulating the production of insulin-like growth factor 1 (IGF-1) in the liver and other tissues.
A peptide hormone primarily produced by the liver in response to stimulation by growth hormone (GH). IGF-1 plays a key role in promoting cell growth, differentiation, and tissue repair, especially during childhood and adolescence. It mimics some of the actions of insulin and is essential for mediating many of the growth-promoting effects of GH.
A peptide hormone that functions primarily as an inhibitory regulator of hormone secretion. It is produced in several tissues, including the hypothalamus, pancreas (delta cells), and gastrointestinal tract.
A peptide hormone produced by the hypothalamus and released by the posterior pituitary gland. It plays a key role in reproductive and social behaviors.
Also known as vasopressin, it is a peptide hormone produced by the hypothalamus and released by the posterior pituitary gland. It regulates water balance in the body by increasing water reabsorption in the kidneys, thereby reducing urine output and conserving body fluids.
A butterfly-shaped endocrine gland located in the front of the neck, just below the larynx. It produces the hormones thyroxine (T₄) and triiodothyronine (T₃), which regulate metabolism, growth, and development. The gland also secretes calcitonin, which helps regulate calcium levels in the blood.
Thyroid hormone production is controlled by thyroid-stimulating hormone (TSH) from the anterior pituitary, forming part of the hypothalamic-pituitary-thyroid axis.
One of the two primary hormones produced by the thyroid gland, the other being T₄ (thyroxine). Although T₃ is produced in smaller quantities than T₄, it is the more biologically active form and is responsible for regulating metabolism, growth, development, and body temperature.
One of the two primary hormones produced by the thyroid gland, the other being T₃ (triiodothyronine). T₄ is the predominant form of thyroid hormone circulating in the blood, but it is less biologically active than T₃. Most T₄ is converted into T₃ in peripheral tissues such as the liver and kidneys.
Four small endocrine glands located on the posterior surface of the thyroid gland. They produce parathyroid hormone (PTH), which plays a critical role in calcium homeostasis.
A dual-function gland located in the upper abdomen, behind the stomach. It has both exocrine and endocrine roles.
A peptide hormone secreted by the alpha cells of the pancreas. Glucagon raises blood glucose levels by stimulating the liver to convert stored glycogen into glucose (glycogenolysis) and to produce glucose from non-carbohydrate sources (gluconeogenesis). It acts as a counter-regulatory hormone to insulin.
A pair of endocrine glands located above each kidney. Each adrenal gland consists of two distinct regions: the adrenal cortex, which produces steroid hormones such as cortisol, aldosterone, and androgens; and the adrenal medulla, which secretes catecholamines like adrenaline (epinephrine) and noradrenaline (norepinephrine). These hormones help regulate metabolism, immune response, blood pressure, and the body’s response to stress.
The inner region of the adrenal gland, responsible for producing and secreting catecholamines, primarily epinephrine (adrenaline) and norepinephrine (noradrenaline). These hormones are released in response to stress and help initiate the "fight-or-flight" response by increasing heart rate, blood pressure, and energy availability. The adrenal medulla functions as part of the sympathetic nervous system.
Also known as adrenaline, epinephrine is a catecholamine hormone and neurotransmitter produced by the adrenal medulla. It plays a central role in the body's "fight-or-flight" response by increasing heart rate, dilating airways, enhancing blood flow to muscles, and promoting the breakdown of glycogen to glucose for quick energy.
Also called noradrenaline, norepinephrine is a catecholamine that functions both as a hormone and a neurotransmitter. It is produced by the adrenal medulla and sympathetic nerve endings. Norepinephrine increases blood pressure by constricting blood vessels and is involved in alertness, arousal, and the stress response.
A group of related hormones and neurotransmitters that include epinephrine, norepinephrine, and dopamine. These compounds are derived from the amino acid tyrosine and are involved in the body's response to stress, regulating heart rate, blood pressure, and metabolism.
The outer region of the adrenal gland, responsible for producing steroid hormones. It consists of three zones: the zona glomerulosa (produces aldosterone), zona fasciculata (produces cortisol), and zona reticularis (produces androgens). These hormones regulate metabolism, immune function, blood pressure, and sexual development.
A class of G protein-coupled receptors that respond to catecholamines such as norepinephrine and epinephrine. These receptors are primarily involved in vasoconstriction, pupil dilation, and other sympathetic nervous system responses.
A class of G protein-coupled receptors that respond to catecholamines such as epinephrine and norepinephrine. These receptors are involved in the regulation of heart rate, smooth muscle relaxation, and metabolic processes.
A class of steroid hormones produced by the adrenal cortex, primarily involved in regulating electrolyte and fluid balance. The most well-known mineralocorticoid is aldosterone, which promotes sodium retention and potassium excretion by the kidneys, helping to maintain blood pressure and volume.
Steroid hormones produced by the adrenal cortex that influence metabolism, immune response, and stress adaptation. The primary glucocorticoid is cortisol, which increases blood glucose levels, suppresses inflammation, and helps the body respond to physical and emotional stress.
A group of steroid hormones that regulate the development and maintenance of male characteristics. Although produced in greater amounts by the testes, androgens such as testosterone are also secreted by the adrenal cortex and ovaries. They influence muscle mass, libido, and secondary sexual traits.
A class of steroid hormones primarily produced by the ovaries, with smaller amounts secreted by the adrenal glands and other tissues. Estrogens regulate the development of female secondary sexual characteristics, reproductive function, and menstrual cycle. The most prominent estrogen is estradiol.
An enzyme secreted by the juxtaglomerular cells of the kidneys in response to low blood pressure, reduced sodium levels, or sympathetic nervous system stimulation. Renin initiates the renin-angiotensin-aldosterone system (RAAS) by cleaving angiotensinogen into angiotensin I, ultimately helping regulate blood pressure and fluid balance.
A plasma protein produced by the liver that serves as the precursor to angiotensin I. When acted upon by renin, angiotensinogen is converted into angiotensin I, beginning a cascade that leads to vasoconstriction and increased blood pressure.
An inactive precursor formed when renin cleaves angiotensinogen. Angiotensin I is converted into the active hormone angiotensin II by the enzyme angiotensin-converting enzyme (ACE), which plays a key role in blood pressure regulation.
An enzyme primarily found in the lungs and vascular endothelium that converts angiotensin I into angiotensin II, a potent vasoconstrictor. ACE also degrades bradykinin, a vasodilator, thereby contributing to increased blood pressure.
A potent vasoconstrictor formed from angiotensin I through the action of angiotensin-converting enzyme (ACE). Angiotensin II increases blood pressure by narrowing blood vessels and stimulates the release of aldosterone from the adrenal cortex. It also promotes thirst and the release of antidiuretic hormone (ADH), contributing to fluid retention and blood pressure regulation.
A steroid hormone produced by the zona glomerulosa of the adrenal cortex. Aldosterone plays a key role in maintaining blood pressure and electrolyte balance by promoting sodium reabsorption and potassium excretion in the kidneys. Its release is primarily stimulated by angiotensin II, elevated potassium levels, and ACTH.
A glucocorticoid hormone produced by the zona fasciculata of the adrenal cortex. Cortisol plays a vital role in the body's response to stress by increasing blood glucose levels, enhancing metabolism of fats, proteins, and carbohydrates, and suppressing inflammation and immune responses. Its secretion is regulated by the hypothalamic-pituitary-adrenal (HPA) axis and follows a diurnal rhythm, peaking in the early morning.
The male gonads responsible for producing sperm and secreting male sex hormones, primarily testosterone. Located in the scrotum, the testes consist of seminiferous tubules (where spermatogenesis occurs) and interstitial (Leydig) cells that produce androgens. They play a central role in male reproductive function and secondary sexual characteristics.
The female gonads that produce ova (eggs) and secrete female sex hormones, including estrogens and progesterone. Located on either side of the uterus, the ovaries contain follicles at various stages of development and are responsible for regulating the menstrual cycle, fertility, and the development of female secondary sexual characteristics.
The primary male sex hormone, classified as an androgen. It is produced mainly by the testes and in smaller amounts by the adrenal cortex. Testosterone plays a key role in the development of male reproductive tissues, secondary sexual characteristics (such as increased muscle mass and body hair), and the regulation of libido and sperm production.
A steroid hormone primarily produced by the corpus luteum in the ovary after ovulation, and in smaller amounts by the adrenal glands and placenta during pregnancy. Progesterone prepares the endometrium (lining of the uterus) for potential implantation of a fertilized egg, supports early pregnancy, and helps regulate the menstrual cycle. It also plays a role in modulating immune responses and maintaining pregnancy.
The most potent and predominant form of estrogen in non-pregnant, reproductive-age females. Produced mainly by the ovaries, estradiol plays a central role in regulating the menstrual cycle, maintaining reproductive tissues, and supporting bone and cardiovascular health.
A weaker form of estrogen that is primarily produced in adipose (fat) tissue and the adrenal glands. Estrone becomes the dominant estrogen after menopause and can be converted into estradiol or estriol depending on physiological needs.
The weakest of the three major estrogens, estriol is produced in significant amounts during pregnancy by the placenta. It plays a role in maintaining pregnancy and preparing the body for childbirth, but has minimal effects outside of gestation.
A chronic condition characterized by decreased bone mass and deterioration of bone tissue, leading to increased fragility and risk of fractures. It occurs when bone resorption outpaces bone formation, often due to aging, hormonal changes (especially reduced estrogen levels in postmenopausal women), nutritional deficiencies, or lack of physical activity.